Explore VUEWAY®
(gadopiclenol)
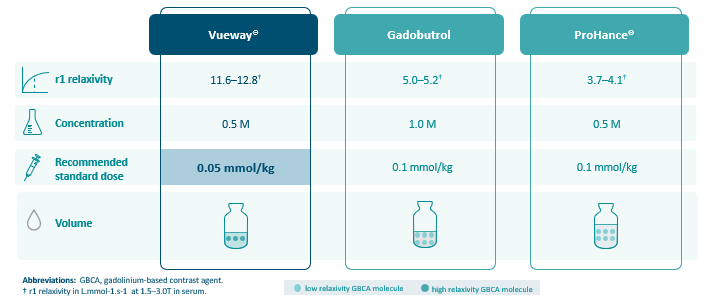
Contrast enhancement at least non-inferior at half the gadolinium dose (0.05 mmol/kg)
vs. a macrocyclic GBCA at a dose of 0.1 mmol/kg in approved indications.1
A lower gadolinium dose for similar visualization can help address the unmet medical need to minimize Gd exposure per MRI procedure and reduce the overall lifetime dose.10,11
References
1VUEWAY® SmPC 2023 (EMA).
2 Loevner L, et al. Invest Radiol. 2023 May;1;58(5):307-313.
3 Kuhl C, et al. Radiol. 2023 Jul;308(1).
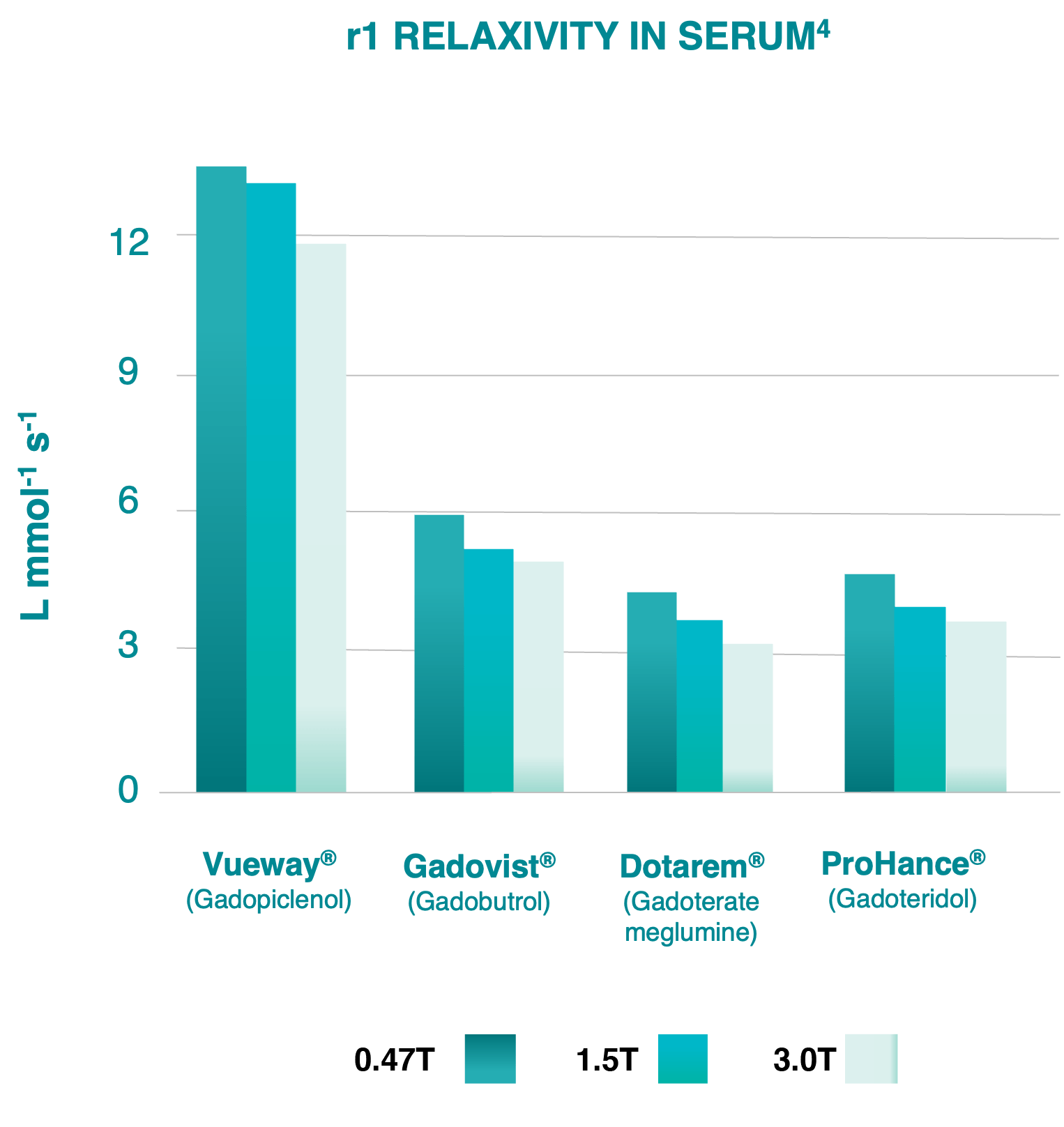
4 Robic C, Port M, Rousseaux O, et al. Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate with High T1 Relaxivity. Invest Radiol. 2019 Aug;54: 475-484.
5 GADOVIST® (gadobutrol) SmPC, 07/2022.
6 ProHance® (gadoteridol) SmPC UK, Nov 2021.
7 DOTAREM® (gadoterate meglumine) SmPC 07/2022.
8 CLARISCAN™ (gadoterate meglumine) SmPC UK, 05/2023.
9 Tweedle MF, Kanal E and Muller R. Considerations in the Selection of a New Gadolinium-Based Contrast Agent. Applied Radiology. Supplement May 2014.
10 Runge VM and Heverhagen, JT. Advocating the Development of Next-Generation High-Relaxivity Gadolinium Chelates for Clinical Magnetic Resonance. Invest Radiol. 2018 Jul;53(7):381-389.
11 Lancelot E, Raynaud JS, Desché P. Current and Future MR Contrast Agents: Seeking a Better Chemical Stability and Relaxivity for Optimal Safety and Efficacy. Invest Radiol. 2020 Sep;55(9):578-588.
12 Bendszus M, et al. Invest Radiol. 2020;55(3):129-137.
13 Bradu A, et al. Invest Radiol. 2021;56(8):486-493.
14 Funck-Brentano C, et al. Br J Clin Pharmacol. 2020;86(11):2174-2181.

