MultiHance®: High-relaxivity GBCA
labelled as a liver-specific contrast agent†
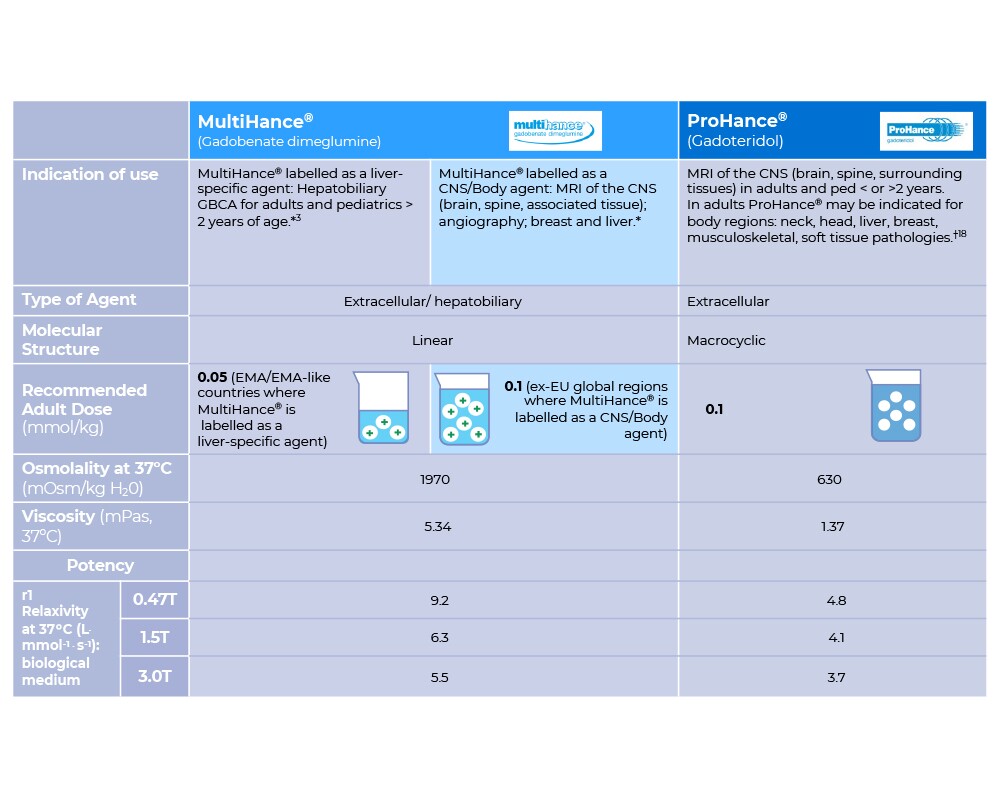
A premium liver-specific GBCA: Dual properties of high relaxivity and hepatocyte-specific uptake help ensure accurate liver diagnostics in both dynamic and delayed imaging at a reduced gadolinium dose (0.05mmol/kg) compared to macrocyclic agents.1-3
†MultiHance® approved indications and/or recommended dose can vary from country to country, e.g. in EU (EMA) labelled as a liver-specific agent, in US (FDA) as a general use GBCA.
ProHance®: A well-established macrocyclic GBCA
with proven stability and safety‡4-6
A trusted multi-use macrocyclic GBCA for adults and pediatric patients, offering high stability,6 a proven safety profile and established diagnostic efficacy.7-9 Indicated for MRI of the CNS, including children of any age (from term neonates).9,10
‡ProHance® approved indications can vary from country to country.
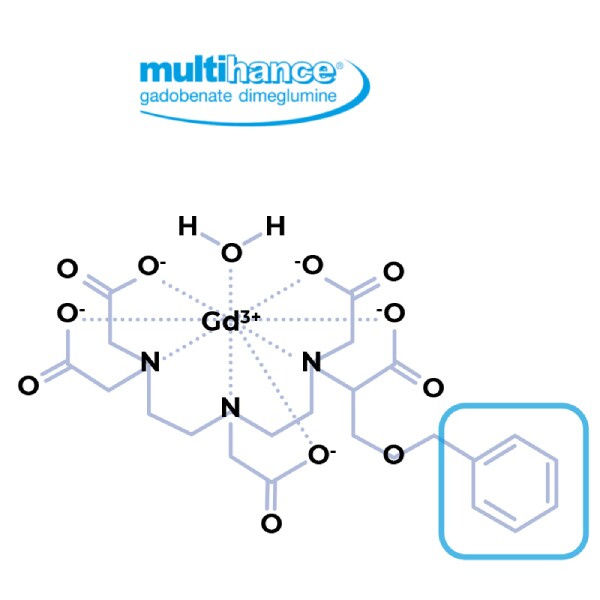
Benzol side chain allows hepatocellular uptake of gadobenate dimeglumine to provide additional information of hepatocyte functionality.2
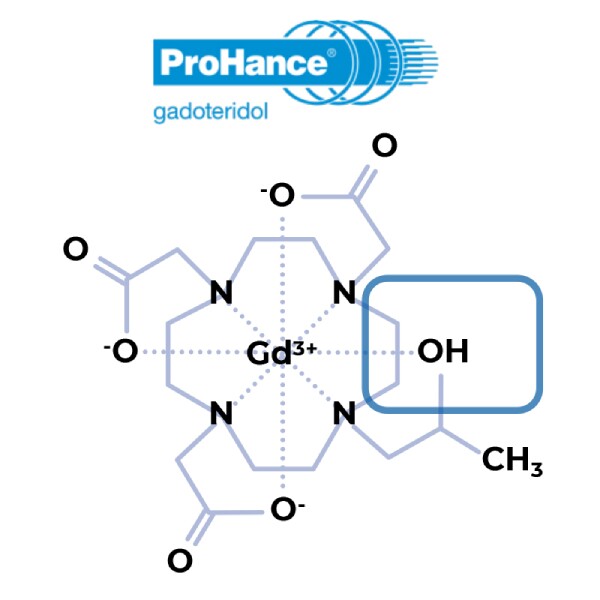
Nonionic and macrocyclic, with unique hydroxyl-containing arm to increase stability.11
360° MRI offering to address all your needs1,9
Advanced safety features
designed to promote patient-centric care

Tilt lockout feature minimizes risk of air embolisms only when the injector head is tilted in the down position.
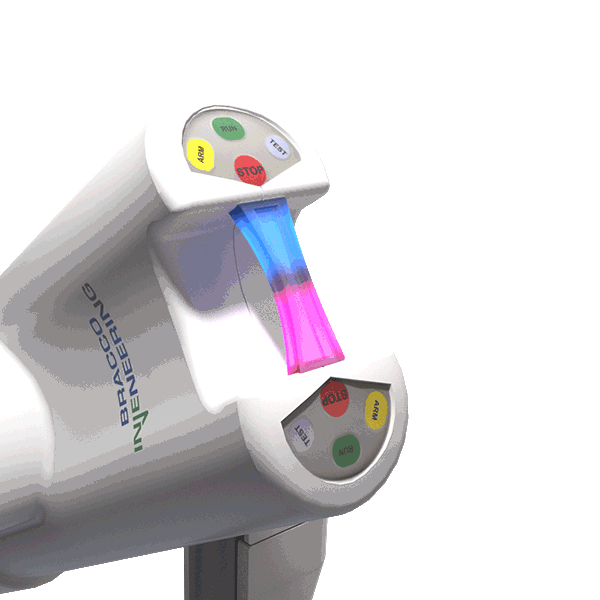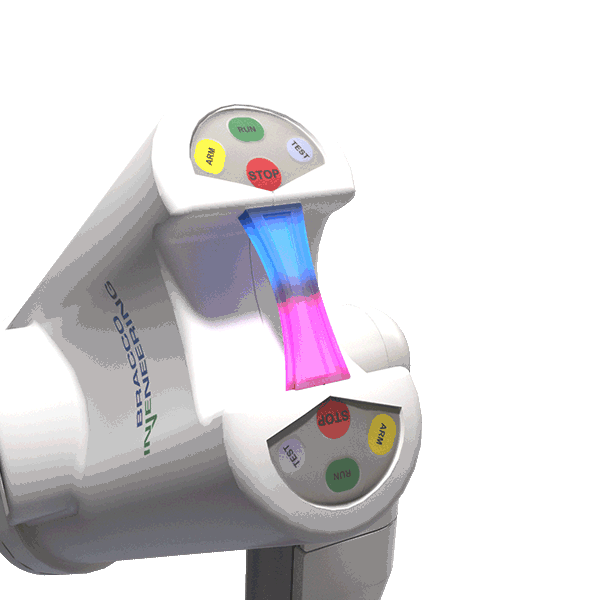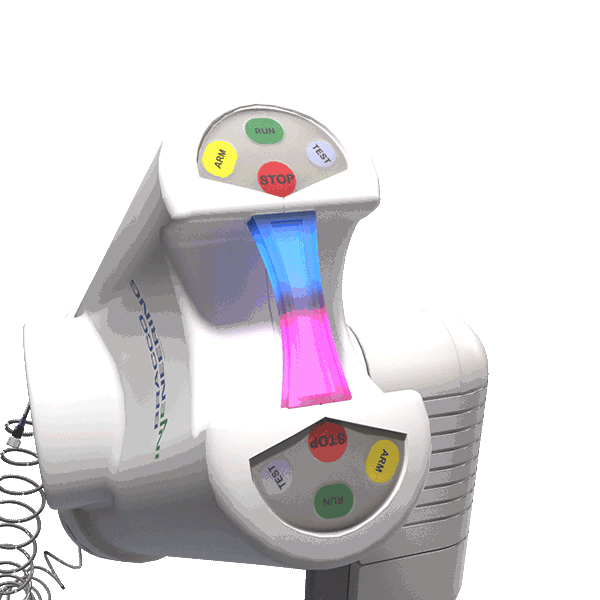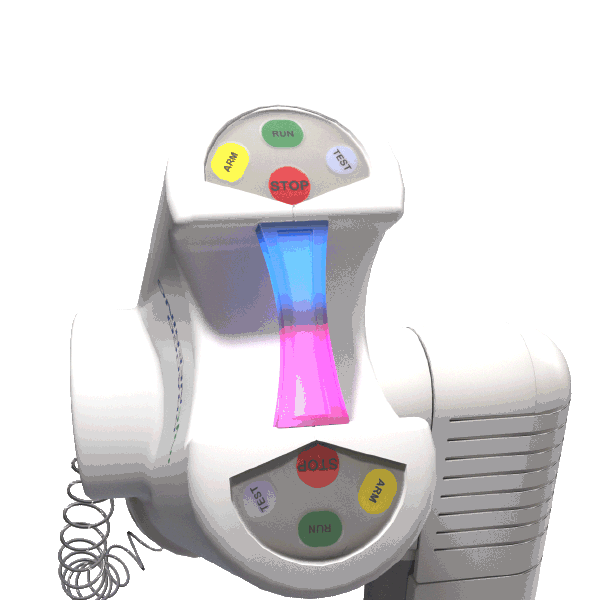
The LED illuminating handle indicates when contrast and saline are in motion.

The eGFR calculator is intended to assist in determining whether the patient’s renal function is consistent with the safe administration of intravenous (IV) contrast.*
eGFR=estimated glomerular filtration rate.
eGFR is not intended to act as a substitute for a physician's diagnosis of conditions, which may preclude the administration of IV contrast or the patient’s ability to accept the procedure.

Pop-up messages alert staff to overpressure during keep vein open (KVO).
Innovative designs
that increase efficiencies and streamline workflow
Remote Interface
User-friendly remote interface for easy viewing and programming protocols
Dual Initialize-and-Replace Syringe
Improves clinical workflow and increases productivity
Compact, Rotating Head
Provides more operational flexibility in small spaces
References
- MultiHance® SmPC 2021.
- Aime S and Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009 Dec;30(6):1259-67.
- Frydrychowicz A et al. Hepatobiliary MR Imaging with Gadolinium-Based Contrast Agents, JMRI 2012;5:492-511.
- Morgan DE et al. Assessment of Adverse reaction Rates during Gadoteridol-enhanced MR Imaging in 28078 Patients. Radiology 2011;259(1):109-116.
- Cho SB et al. Prospective Multicenter Study of the Safety of Gadoteridol in 6163 Patients. J Magn Reson Imaging 2020;51(3):861-868.
- Laurent S et al. Comparative study of the physico- chemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Med. Mol. Imaging 2006;1:128–137.
- Maravilla K et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (the TRUTH study). Am J Neuroradiol. 2015;36(1):14-23.
- Del Poggio A et al. Diagnostic efficacy and safety of gadoteridol compared to gadobutrol and gadoteric acid in a large sample of CNS MRI studies at 1.5T. J Neuroradiol. 2022 Jan;49(1):73-79.
- ProHance® SmPC 2021.
- Shah CC, et al, Safety and diagnostic efficacy of gadoteridol for magnetic resonance imaging of the brain and spine in children 2 years of age and younger. Pediatric Radiol. 2021;51: 1895–1906.
- Tweedle MF. The ProHance story: the making of a novel MRI contrast agent. Eur Radiol. 1997;7 Suppl 5:225-30.