Reasons for VUEWAY's high relaxivity:
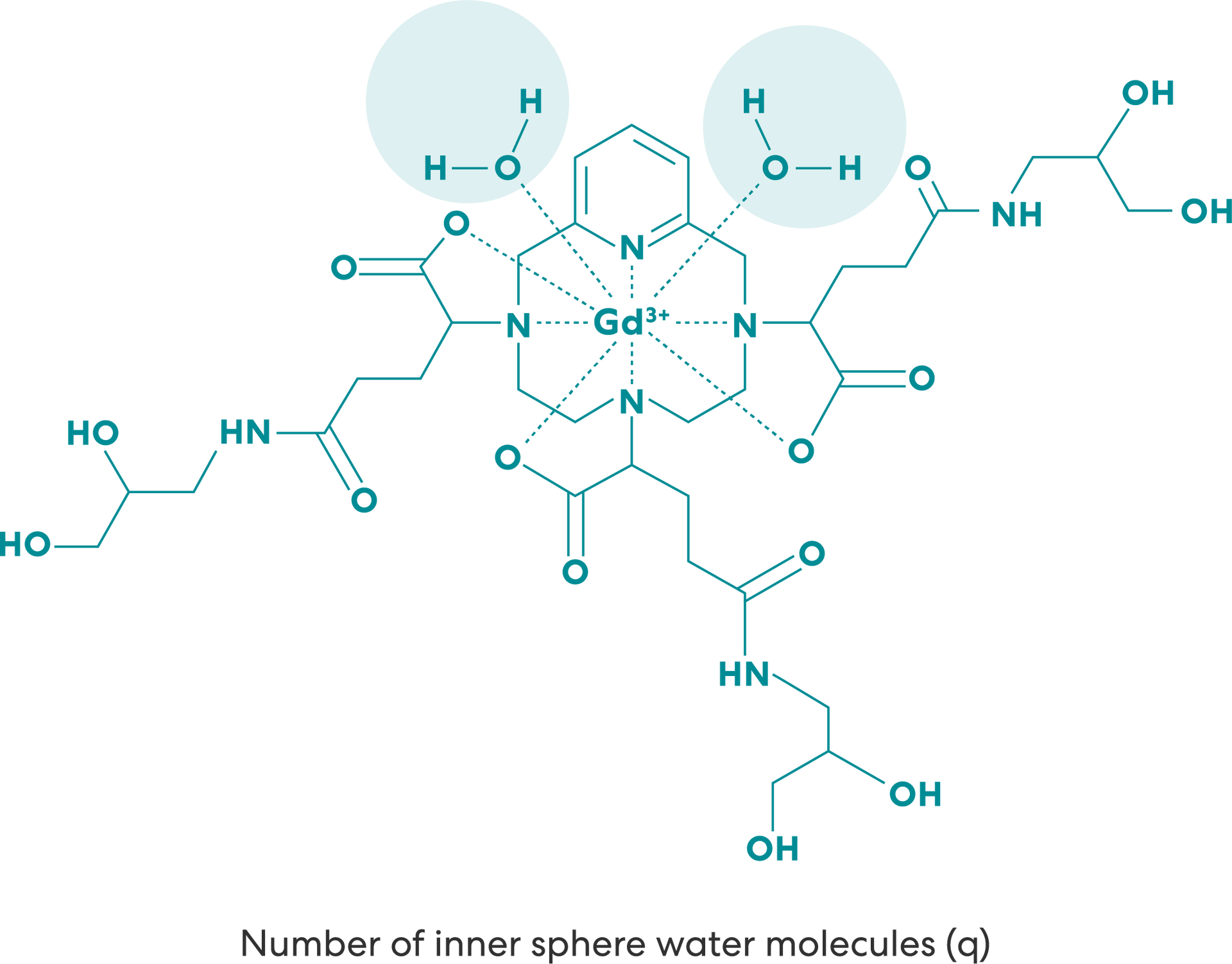
The VUEWAY molecule permits the coordination of two water molecules in the inner sphere (q=2) versus all other gadolinium-based contrast agents (GBCAs) (q=1).
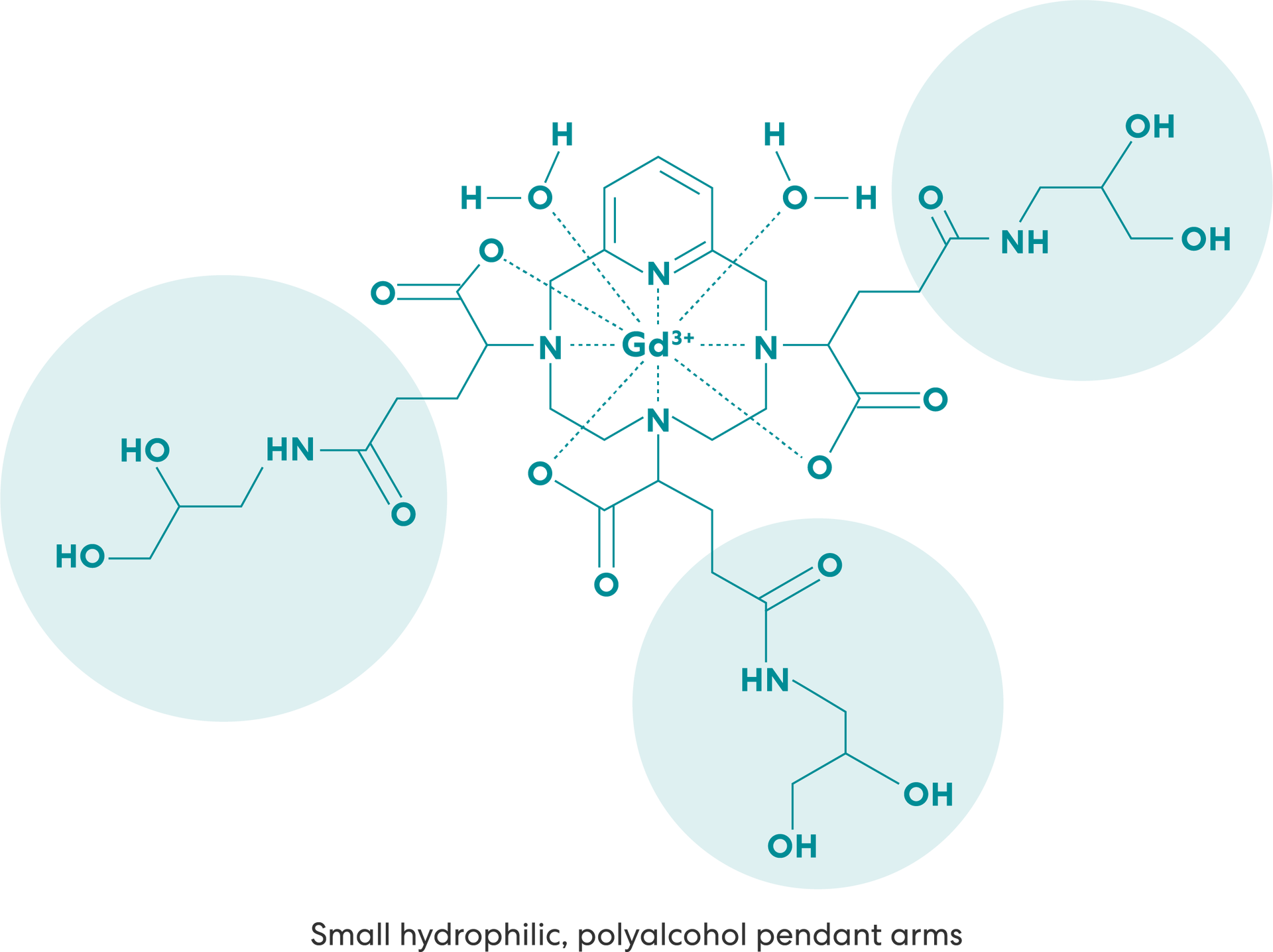
The VUEWAY molecule was designed with small hydrophilic, polyalcohol pendant arms, which reduce the rotational diffusion. This results in slower molecular rotation versus lower-relaxivity GBCAs.
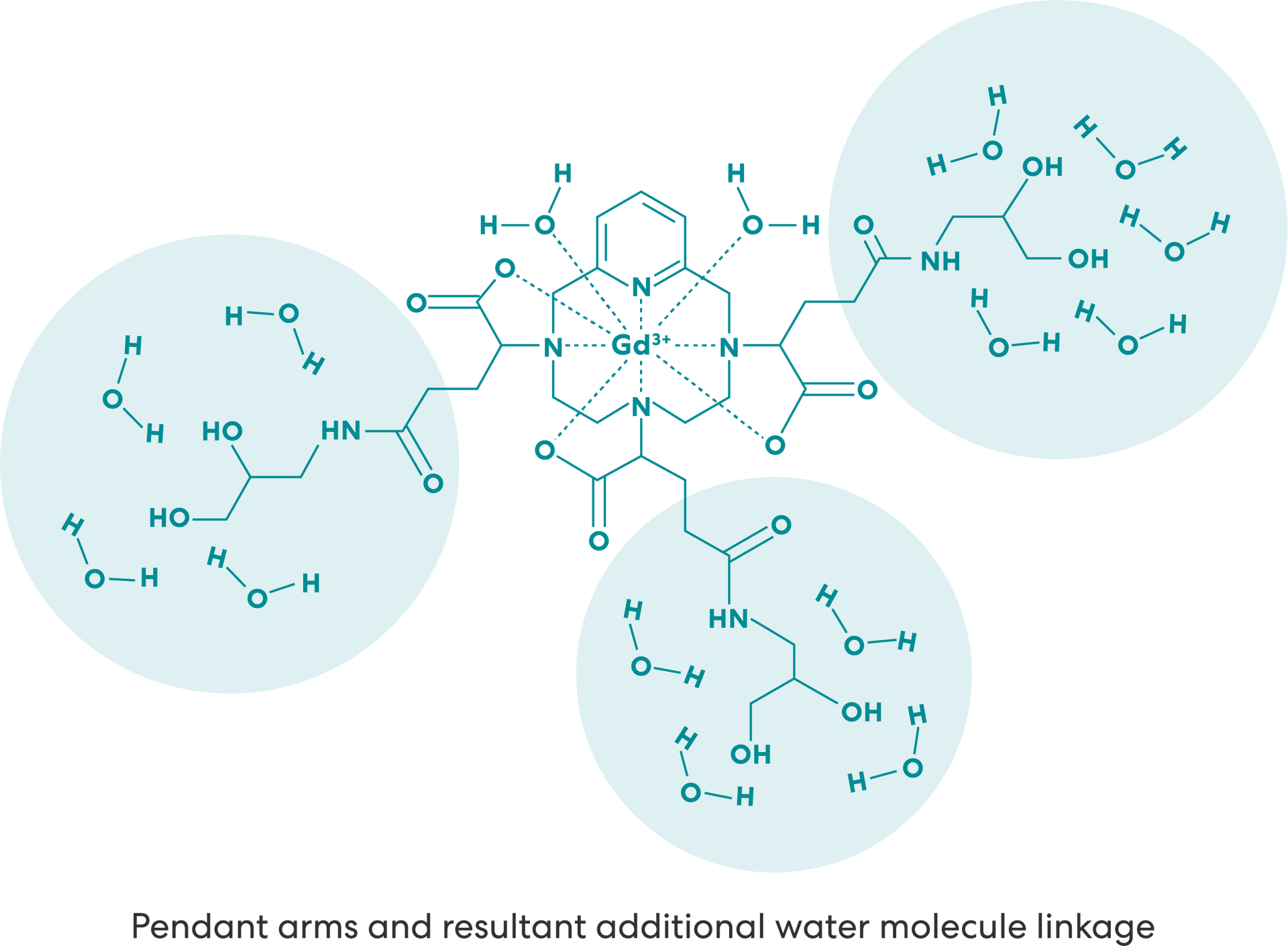
The hydrophilic pendant arms increase the number of water molecules linked by the second sphere mechanism, further increasing the r1 relaxivity.
The result?
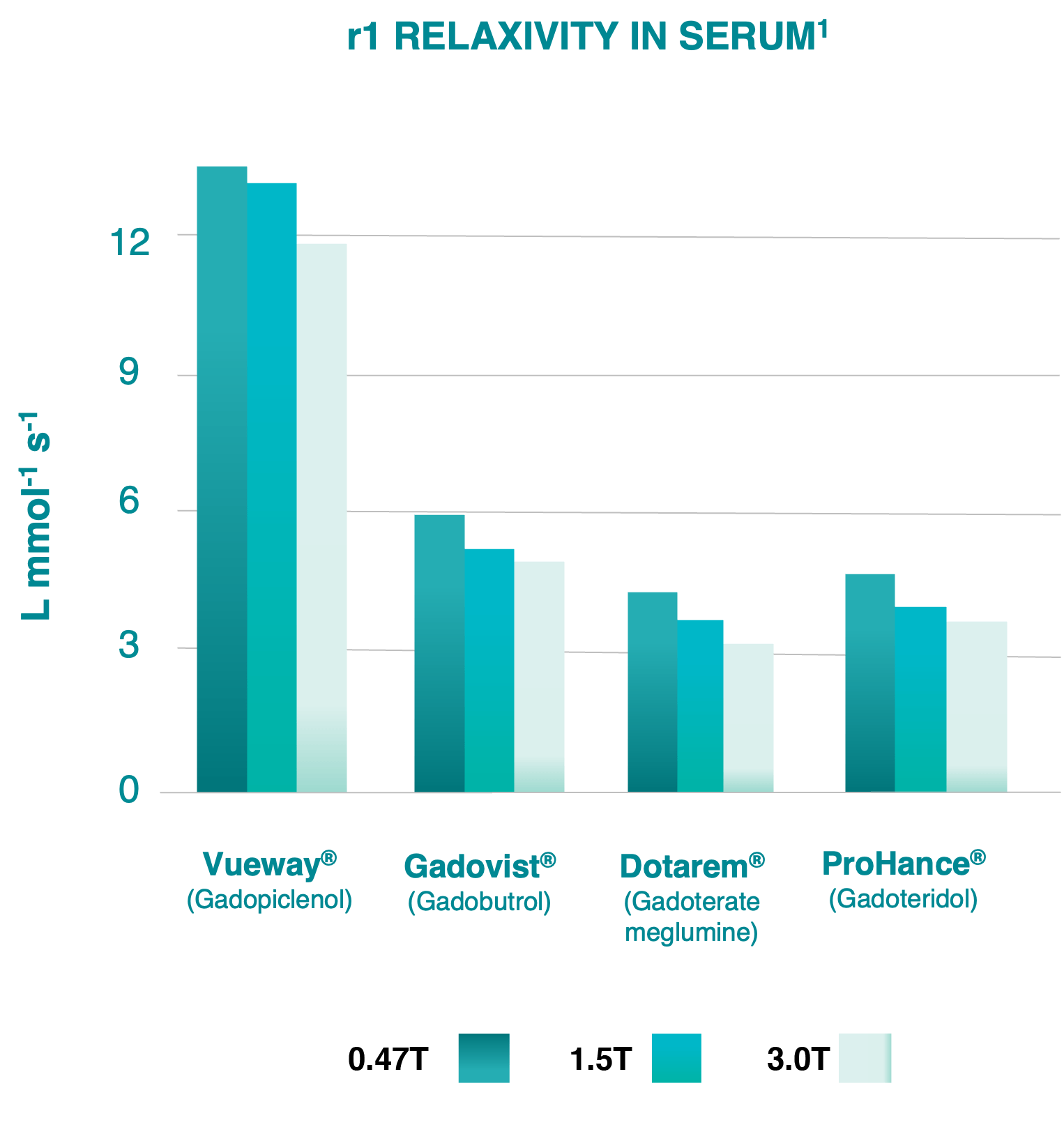
Gadopiclenol: The highest relaxivity of
all GBCAs today.¹
References
1 Robic C, Port M, Rousseaux O, et al. Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium ChelateWith High T1 Relaxivity. Invest Radiol 2019; 54: 475-484.
