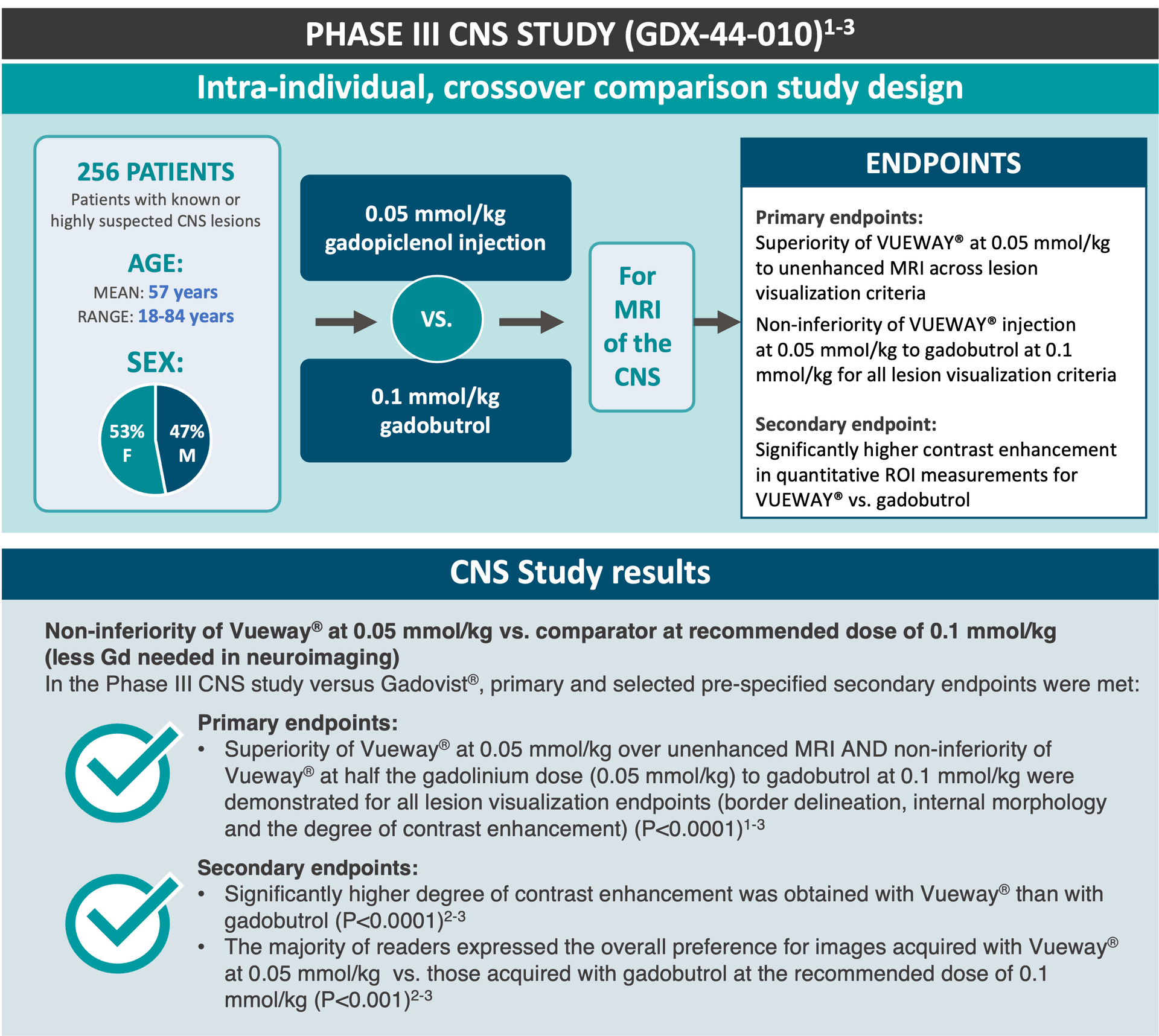
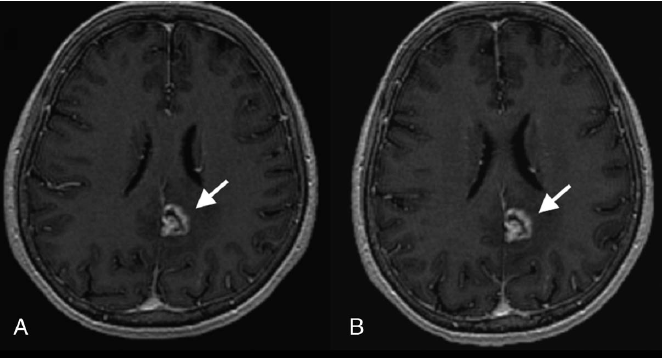
Clinical case from the dose-finding study of VUEWAY®: 65-year-old female patient with brain metastasis from lung cancer. A similar level of contrast enhancement, lesion border delineation, and visualization of lesion internal morphology were obtained with
0.05 mmol/kg VUEWAY® (A) vs. 0.1 mmol/kg MultiHance®* (B).4
*The study was performed in US with dosing and indications approved by FDA (note: not applicable in EU).
References
1 VUEWAY® (gadopiclenol) SmpC, EMA, 10/2023.
2 Loevner LA, Kolumban B, Hutóczki G, et al. Efficacy and Safety of Gadopiclenol for Contrast-Enhanced MRI of the Central Nervous System: The PICTURE Randomized Clinical Trial. Invest Radiol. 2022 Dec 19. doi: 10.1097/RLI.0000000000000944.
3 Groden C, Pichiecchio A, Kolumban B, et al. Efficacy and Safety Of Gadopiclenol For Central Nervous System (CNS) Magnetic Resonance Imaging (MRI): Results From The Phase III Picture Trial. Neuroradiology. 2022 Aug; 64 (Suppl 1):S113– S114 (ESNR 2022).
4 Bendszus M, Roberts D, Kolumban B, et al. Dose Finding Study of Gadopiclenol, a New Macrocyclic Contrast Agent, in MRI of Central Nervous System. Invest Radiol. 2020 Mar;55(3):129-137.
